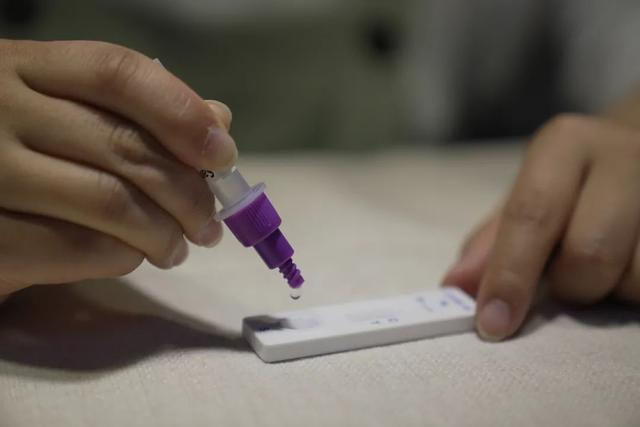
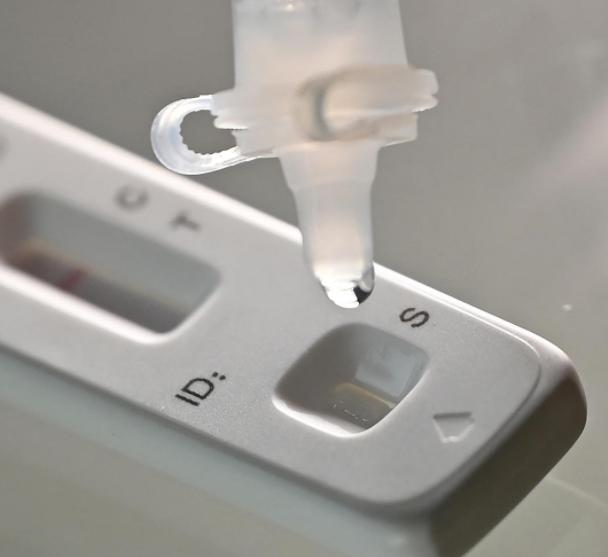
12-kii Maarsoth 2022, ahNMPA (SFDA) waxay soo saartay ogaysiis ansixinaya beddelka codsiga is-baadhista COVID-19 ee alaabta antigen-ka ee Nanjing Vazyme BiotechCo., Ltd, Beijing Jinwofu Bioengineering TechnologyCo., Ltd, Shenzhen Huada Yinyuan Pharmaceutical Technology Co., Ltd, Hal qayb oo ka mid ah Guangzhou Wondfo Biotech Co., Ltd iyoBHal qayb oo ka mid ah eijing Savant Biotechnology Co., Ltd(Huaketai).Shan alaab oo COVID-19 antigen ah ayaa la bilaabay.
March 11th, 2022, NHC waxay ku dhawaaqday in, si loo sii wanaajiyo istiraatijiyadda baaritaanka Novel Coronavirus oo loogu adeego baahiyaha ka hortagga iyo xakameynta COVID-19, Kooxda Dhameystiran ee ka hortagga iyo xakameynta wadajirka ah ee Golaha Gobolka ayaa go'aansaday in lagu daro. Tijaabada antigen-ka si loo baadho aashitada nucleic-ka ah oo la sameeyay "Protocol-ka Codsiga ee Novel Coronavirus Antigen Detection (Trial)"
Nidaamku wuxuu qeexayaa dadka lagu dabaqi karo baaritaanka antigen-ka:
Marka hore, kuwa booqda xarumaha caafimaadka aasaasiga ah oo leh calaamado ay ka mid yihiin mareenka neefsiga iyo qandhada 5 maalmood gudahooda ee bilawga calaamadaha;
Marka labaad, shaqaalaha fiirsashada karantiilka, oo ay ku jiraan indho-indhaynta karantiilka guriga, xidhiidh dhow iyo xidhiidh hoose, indho-indhaynta karantiilka gelitaanka, aagga xakamaynta iyo shaqaalaha goobta xakamaynta;
Midda saddexaad waa dadka deegaanka ee u baahan is-ogaanshaha antigen-ka.
Talooyin: Ogaanshaha Antigen-ka waa kabitaan muhiim ah oo lagu ogaanayo aashitada nucleic, laakiin natiijooyinka is-oggolaanshaha antigen looma isticmaali karo aasaaska ogaanshaha cudurka
Waqtiga boostada: Mar-22-2022